
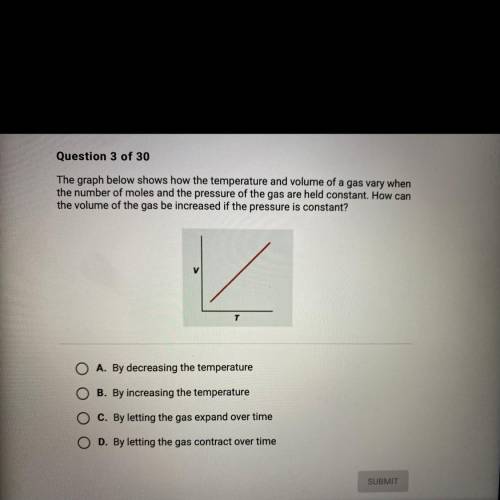
The graph below shows how the temperature and volume of a gas vary when
the number of moles and the pressure of the gas are held constant. How can
the volume of the gas be increased if the pressure is constant?
A. By decreasing the temperature
B. By increasing the temperature
C. By letting the gas expand over time
D. By letting the gas contract over time

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1
You know the right answer?
The graph below shows how the temperature and volume of a gas vary when
the number of moles and the...
Questions





Mathematics, 20.07.2019 03:30


Business, 20.07.2019 03:30










Computers and Technology, 20.07.2019 03:30





