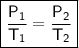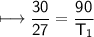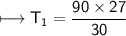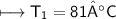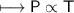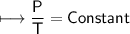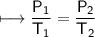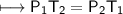Chemistry, 03.08.2021 14:10 Vickyvics4113
The gases in a hair spray can are at a temperature of 27oC and a pressure of 30 lbs/in2. If the
gases in the can reach a pressure of 90 lbs/in2, the can will explode. To what temperature must
the gases be raised in order for the can to explode? Assume constant volume. Show your work.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2


Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
The gases in a hair spray can are at a temperature of 27oC and a pressure of 30 lbs/in2. If the
gas...
Questions




Mathematics, 23.08.2021 22:10


Mathematics, 23.08.2021 22:10

English, 23.08.2021 22:10

English, 23.08.2021 22:10









Geography, 23.08.2021 22:20