
Chemistry, 03.08.2021 20:10 isamar4348
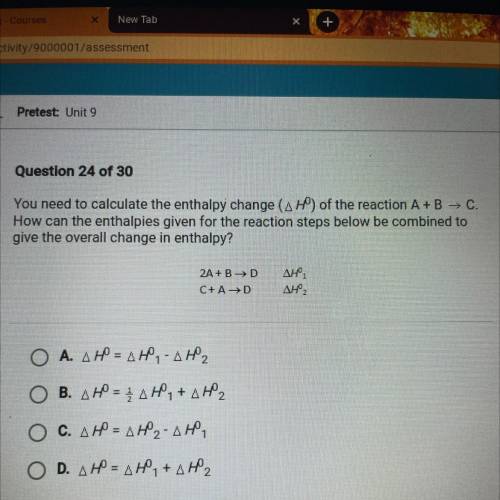
You need to calculate the enthalpy change (AH) of the reaction A+B → C.
How can the enthalpies given for the reaction steps below be combined to
give the overall change in enthalpy?
2A + BD
C+
AD
ΔΗ,
AHZ

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
You need to calculate the enthalpy change (AH) of the reaction A+B → C.
How can the enthalpies give...
Questions



Mathematics, 09.08.2019 00:20





Biology, 09.08.2019 00:20

Biology, 09.08.2019 00:20









Biology, 09.08.2019 00:20




