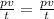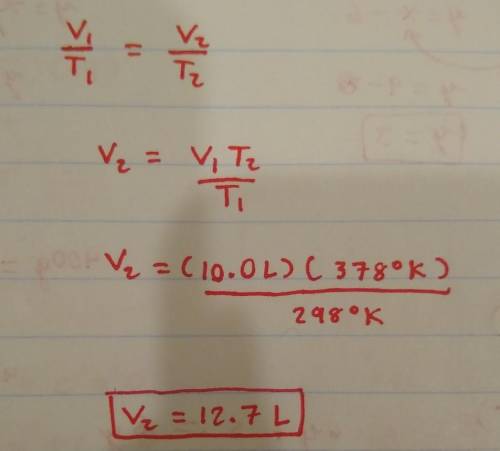Chemistry, 04.08.2021 03:40 acrespo3425
a fixed amount of gas at 25.0 degrees Celsius occupies a volume of 10.0 L when the pressure is 629 torr. Use Charles law to calculate the volume the gas will occupy when the temperature is increased to 105 degrees Celsius while maintaining the pressure at 629 torr?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
a fixed amount of gas at 25.0 degrees Celsius occupies a volume of 10.0 L when the pressure is 629 t...
Questions

Spanish, 18.03.2021 01:40


Mathematics, 18.03.2021 01:40





English, 18.03.2021 01:40


History, 18.03.2021 01:40



History, 18.03.2021 01:40

Spanish, 18.03.2021 01:40






Mathematics, 18.03.2021 01:40