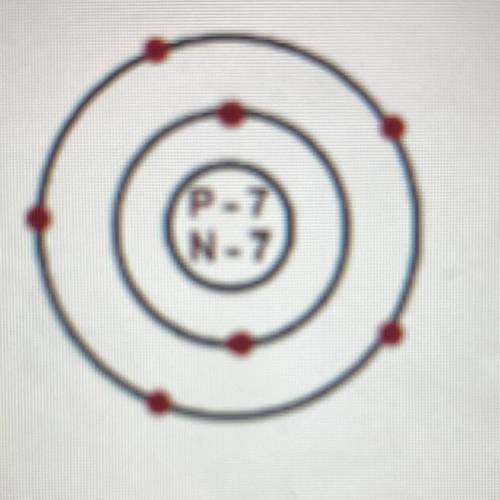
Explanation:
Hi there!
From the figure;
We can figure out that the atomic number is 7.
So;
Option A: 1s² 2s² 2p²
Sum of the powers of variable is just 6. So, it's not answer.
Option B: 1s²2s²2p³
Sum of the powers of variable is 7. So it's the answer
Option C: 1s²2s²2p⁴
Sum of the powers of variable is 8. So, it's not answer.
Option D: 1s²2s²2p⁵
Sum of the powers of variable is 9. So, it's not the correct answer.
Therefore, answer is option B.
Hope it helps!