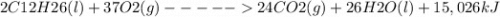Chemistry, 06.08.2021 14:00 leylaanddade
Consider the following thermochemical reaction for kerosene:
2 C12H26(l) + 37 O2(g) 24 CO2(g) + 26 H2O(l) + 15,026 kJ
(a) When 21.3 g of CO2 are made, how much heat is released?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3

Chemistry, 23.06.2019 09:00
Which question could be best answered using the process of scientific inquiry? do different plates have different rock compositions? why did it take so long to develop the theory of plate tectonics? what are different cultural myths caused by plate tectonics? do plates move intentionally to cause volcanic eruptions?
Answers: 3
You know the right answer?
Consider the following thermochemical reaction for kerosene:
2 C12H26(l) + 37 O2(g) 24 CO2(g) + 2...
Questions

Chemistry, 11.04.2022 02:50


Biology, 11.04.2022 03:00


Mathematics, 11.04.2022 03:00


Biology, 11.04.2022 03:10




Mathematics, 11.04.2022 03:10

Business, 11.04.2022 03:20

Mathematics, 11.04.2022 03:20

Mathematics, 11.04.2022 03:20


Mathematics, 11.04.2022 03:20

Mathematics, 11.04.2022 03:30


Mathematics, 11.04.2022 03:30