Chemistry, 08.08.2021 06:00 destinywashere101
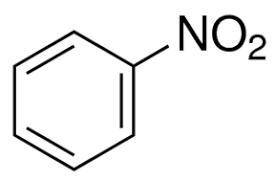
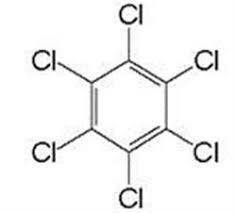
Suppose 'A' is a liquid aromatic compound with molecular weight 78 and burns with sooty flame. a. Give the name of the compound 'A' b. write the molecular structure of 'A' C. What is the product when 'A' is treated with ? i. conc. HNO3 with conc. H2SO4 as catalyst ii. Halogen (cl2)in presence of sunlight and mention the use of the product obtained

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 20:30
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
Suppose 'A' is a liquid aromatic compound with molecular weight 78 and burns with sooty flame. a. Gi...
Questions















Mathematics, 18.05.2021 19:20