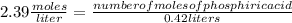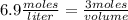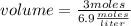Chemistry, 09.08.2021 01:20 ayoismeisjuam
What volume of 6.9 M NaOH is needed to completely titrate 0.42 L of 2.39 M phosphoric acid according to
the equation:
H3PO4(aq) + 3NaOH(aq) + Na3PO4(aq) + 3H2O(aq)
A) O 0.05 L
B) O6.93 L
C) O0.44 L
D) 03.01 L
E) 436.43 L


Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1

Chemistry, 23.06.2019 10:00
The image shows the process of which is used in nuclear power plants. photo attached
Answers: 1

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3

Chemistry, 23.06.2019 14:10
What is true according to the second law of thermodynamics
Answers: 1
You know the right answer?
What volume of 6.9 M NaOH is needed to completely titrate 0.42 L of 2.39 M phosphoric acid according...
Questions

Mathematics, 09.02.2021 20:30



Mathematics, 09.02.2021 20:30





English, 09.02.2021 20:30

Physics, 09.02.2021 20:30




Mathematics, 09.02.2021 20:30






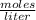 .
.