
Chemistry, 09.08.2021 14:00 ilovecatsomuchlolol
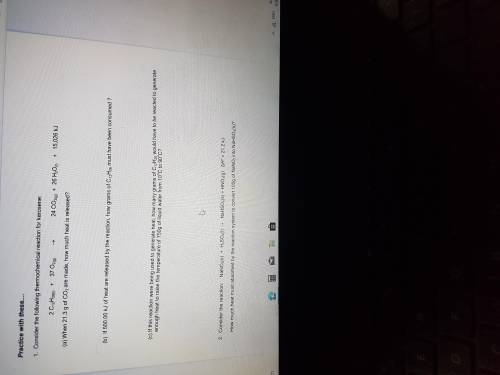
Consider the following thermochemical reaction for kerosene:
2CH12H26(l)+37O2(g).24CO2(g)+26H2O( l)+15,026kj
A. When 21.3g of CO2 are made, how much heat is released ?
B. If 500.00kj of heat are released by the reaction, how many grams of C12H26 must be produced?
C. If this reaction were to being used to generate heat , how many grams of C12H26 would have to be reacted to generate enough heat to raise the temperature of 750g of liquid water from 10 degrees Celsius to 90 degrees celsius ?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which of the following is a compound? a.carbon b.oxygen c.hydrogen d.water
Answers: 2

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
Consider the following thermochemical reaction for kerosene:
2CH12H26(l)+37O2(g).24CO2(g)+26H2O( l...
Questions



History, 28.10.2019 13:31

History, 28.10.2019 13:31

Chemistry, 28.10.2019 13:31


Mathematics, 28.10.2019 13:31


Mathematics, 28.10.2019 13:31

History, 28.10.2019 13:31



Mathematics, 28.10.2019 13:31

Geography, 28.10.2019 13:31

Biology, 28.10.2019 13:31

Mathematics, 28.10.2019 13:31



Mathematics, 28.10.2019 13:31

Biology, 28.10.2019 13:31



