
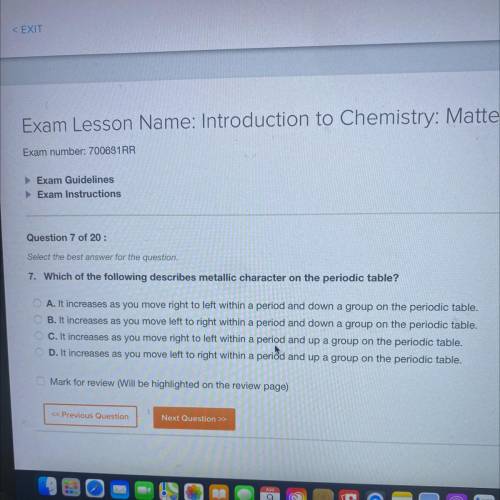
Which of the following describes metallic character on the periodic table?
A. It increases as you move right to left within a period and down a group on the periodic table.
B. It increases as you move left to right within a period and down a group on the periodic table.
C. It increases as you move right to left within a period and up a group on the periodic table.
D. It increases as you move left to right within a period and up a group on the periodic table.
Mark for review (Will be highlighted on the review nane)
help

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the mass in grams of hydrogen gas produced when 14.0 moles of hcl is added to an excess amount of magnesium.
Answers: 3

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3
You know the right answer?
Which of the following describes metallic character on the periodic table?
A. It increases as you m...
Questions

English, 05.05.2020 02:53

Mathematics, 05.05.2020 02:53

Mathematics, 05.05.2020 02:53

Mathematics, 05.05.2020 02:53


History, 05.05.2020 02:53

Computers and Technology, 05.05.2020 02:53





Mathematics, 05.05.2020 02:54



English, 05.05.2020 02:54



Social Studies, 05.05.2020 02:54

Mathematics, 05.05.2020 02:54

History, 05.05.2020 02:54



