
Chemistry, 10.08.2021 07:10 ikramhamideh
Determine what product will be produced at the negative electrode for the following reaction:
2KCl(aq) + 2H20(1) -> H2(g) + Cl2(g) + 2KOH(aq)
A. H2
B. Cl2
с. КОН
D. K

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2


Chemistry, 23.06.2019 13:00
The gram molecular mass or co2 is the same as the gram molecular mass of
Answers: 2

Chemistry, 23.06.2019 13:30
Determine the osmotic pressure at 25 °c of an aqueous solution that is 0.028 m nano3. a) 0.685 atm b) 0.0729 atm c) 1.37 atm d) 0.0364 atm e) 2.06 atm
Answers: 2
You know the right answer?
Determine what product will be produced at the negative electrode for the following reaction:
2KCl(...
Questions




Mathematics, 16.09.2021 01:00

Mathematics, 16.09.2021 01:00

Arts, 16.09.2021 01:00

Mathematics, 16.09.2021 01:00

Health, 16.09.2021 01:00



Geography, 16.09.2021 01:00

Social Studies, 16.09.2021 01:00

Mathematics, 16.09.2021 01:00


Mathematics, 16.09.2021 01:00

English, 16.09.2021 01:00

Business, 16.09.2021 01:00


Mathematics, 16.09.2021 01:00

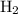 would be produced at the negative electrode.
would be produced at the negative electrode.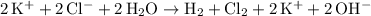 .
.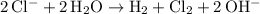 .
.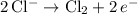 .
.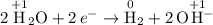 .
. 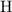 atoms from
atoms from  .)
.)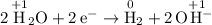 . Hence,
. Hence, 


