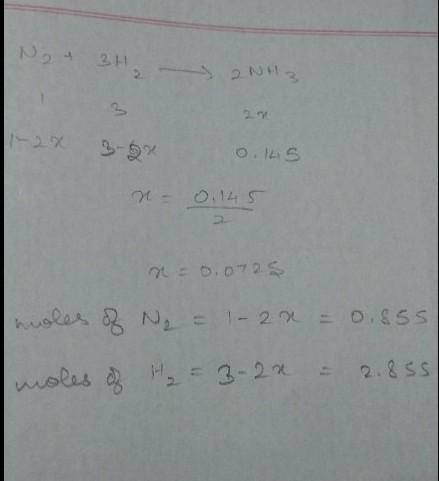Chemistry, 11.08.2021 18:20 maevemboucher78
How many moles of H2 and N2 can be formed by the decomposition of 0.145 mol of ammonia, NH3 ?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
How many moles of H2 and N2 can be formed by the decomposition of 0.145 mol of ammonia, NH3 ?...
Questions


Mathematics, 17.11.2020 23:10




Mathematics, 17.11.2020 23:10


Mathematics, 17.11.2020 23:10



Mathematics, 17.11.2020 23:10


Mathematics, 17.11.2020 23:10



English, 17.11.2020 23:10

History, 17.11.2020 23:10


Mathematics, 17.11.2020 23:10