
Assuming that no equilibria other than dissolution are involved, calculate the concentration of all solute species in each of the following solution of salt in contact with a solution containing a common ion. Show that changes in the initial concentrations:
PbCl2(s) in 0.631 M MgCl2 (MgCl2 is strong electrolyte)
Ksp PbCl2 = 1.6 × 10−5

Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 11:00
Predict the products of the following acid-base reactions, and predict whether the equilibrium lies to the left or to the right of the reaction arrow.part ao2-(aq)+h2o(l)< => express your answer as part of a chemical equation. identify all of the phases in your answer.o2-(aq)+h2o(l) < => oh-(aq)+oh-(aq)part bpredict whether the equilibrium lies to the left or to the right of the equation in previous part.h2o is a stronger acid than oh–, so the equilibrium lies to the right.h2o is a weaker acid than oh–, so the equilibrium lies to the left.h2o is a stronger acid than oh–, so the equilibrium lies to the left.h2o is a weaker acid than oh–, so the equilibrium lies to the right.part cch3cooh(aq)+hs? (aq) < => express your answer as part of a chemical equation. identify all of the phases in your answer.ch3cooh(aq)+hs-(aq) < => h2s(aq)+c2h3o2-(aq)h2s(aq)+c2h3o2-(aq)part dpredict whether the equilibrium lies to the left or to the right of the equation in previous part.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the right.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the left.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the right.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the left.part eno2-(aq)+h2o(l) < => express your answer as part of a chemical equation. identify all of the phases in your answer.no2-(aq)+h2o(l) < => part fpredict whether the equilibrium lies to the left or to the right of the equation in previous part.hno2 is a stronger acid than h2o, so the equilibrium lies to the right.hno2 is a weaker acid than h2o, so the equilibrium lies to the left.hno2 is a stronger acid than h2o, so the equilibrium lies to the left.hno2 is a weaker acid than h2o, so the equilibrium lies to the right.
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
Assuming that no equilibria other than dissolution are involved, calculate the concentration of all...
Questions





History, 09.07.2019 19:30




English, 09.07.2019 19:30



Chemistry, 09.07.2019 19:30


Mathematics, 09.07.2019 19:30






![[Mg^{2+} ] = 0.631 M \\ [Pb] = 1.0 \times 10^{-5} M \\[Cl^{-} ] = 1.26 M](/tpl/images/1407/4448/37a02.png)
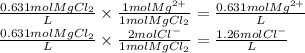
![Ksp = 1.6 \times 10^{-5} = [Pb^{2+} ][Cl^{-} ]^{2} = (x) (1.26+x)^{2}](/tpl/images/1407/4448/5a884.png)
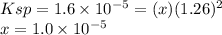
![[Mg^{2+} ] = 0.631 M\\ [Pb] = x = 1.0 \times 10^{-5} M\\[Cl^{-} ] = 1.26+x = 1.26 M](/tpl/images/1407/4448/f1396.png)


