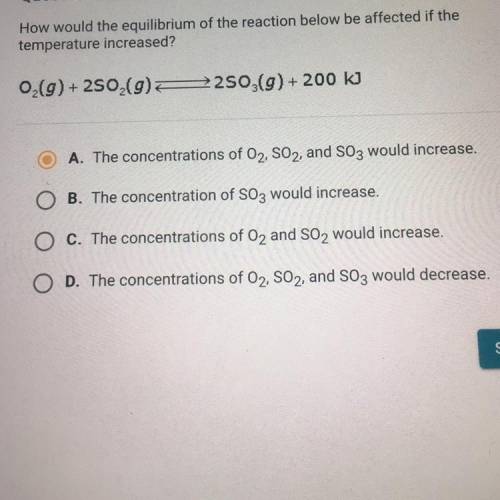
How would the equilibrium of the reaction below be affected if the temperature increased?
...

Chemistry, 12.08.2021 03:20 taminazaka1
How would the equilibrium of the reaction below be affected if the temperature increased?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

You know the right answer?
Questions




Computers and Technology, 30.10.2020 17:00

Mathematics, 30.10.2020 17:00

Health, 30.10.2020 17:00

Biology, 30.10.2020 17:00

History, 30.10.2020 17:00

Mathematics, 30.10.2020 17:00




Mathematics, 30.10.2020 17:10









