
Chemistry, 12.08.2021 18:20 Jasminehenry123
Q1: A stock solution containing Mn+2 ions was prepared by dissolving 1.584 g pure manganese metal in nitric acid and diluting to a final volume of 1.000 L. The following solutions were then prepared by dilution: For solution A, 50.00 mL of stock solution was diluted to 1000.0 mL. For solution B, 10.00 mL of solution A was diluted to 250.0 mL. For solution C, 10.00 mL of solution B was diluted to 500.0 mL. Calculate the concentrations of the stock solution and solutions A, B, and C.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2


You know the right answer?
Q1: A stock solution containing Mn+2 ions was prepared by dissolving 1.584 g pure manganese metal in...
Questions




Social Studies, 08.01.2021 23:00



History, 08.01.2021 23:00


Mathematics, 08.01.2021 23:00





Mathematics, 08.01.2021 23:00

Mathematics, 08.01.2021 23:00

Mathematics, 08.01.2021 23:00

Mathematics, 08.01.2021 23:00

Mathematics, 08.01.2021 23:00


Mathematics, 08.01.2021 23:00

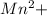 = 1.584 g/55g/mol = 0.0288 moles
= 1.584 g/55g/mol = 0.0288 moles

