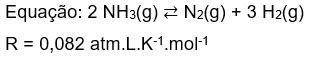Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
4) Considere que em condições de equilíbrio as pressões parciais de NH₃(g), N₂(g) e H₂(g) são respec...
Questions


Social Studies, 27.09.2019 12:00

Geography, 27.09.2019 12:00


History, 27.09.2019 12:00

History, 27.09.2019 12:00

History, 27.09.2019 12:00



Mathematics, 27.09.2019 12:00





Mathematics, 27.09.2019 12:00