
Chemistry, 12.08.2021 20:40 mariahcrook7
4) Considere que em condições de equilíbrio as pressões parciais de NH₃(g), N₂(g) e H₂(g) são respectivamente 18 atm; 6 atm e 12 atm, e essa reação foi realizada a -213 °C. A constante de equilíbrio em termos de concentração (Kc), nas condições acima é: *
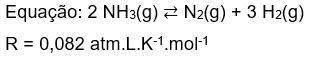

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1


Chemistry, 23.06.2019 09:20
Due tomorrow which would have a lower ph, a 0.1 m solution of a strong base or a weak base? why? which would have a higher ph, a 0.1 m solution of a strong base or a weak base? why?
Answers: 3
You know the right answer?
4) Considere que em condições de equilíbrio as pressões parciais de NH₃(g), N₂(g) e H₂(g) são respec...
Questions

Mathematics, 02.03.2021 06:50

History, 02.03.2021 06:50


Mathematics, 02.03.2021 06:50

Biology, 02.03.2021 06:50

History, 02.03.2021 06:50

Mathematics, 02.03.2021 06:50

History, 02.03.2021 06:50


French, 02.03.2021 06:50




English, 02.03.2021 06:50



Mathematics, 02.03.2021 06:50

Mathematics, 02.03.2021 06:50


Chemistry, 02.03.2021 06:50



