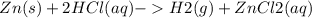A sample of brass weighing 1.203g was analyzed. The zinc in the alloy was reacted with 35.123g of HCl in excess, according to the balanced equation:
Zn (s) + 2HCl(aq) - > H2(g) + ZnCl2 (aq)
After all the zinc reacted, the mass of the remaining solution weighed 36.309g
What was the mass of H2 produced?
What mass of Zn reacted?
What was the percentage of Zn (by mass) in the alloy?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
A sample of brass weighing 1.203g was analyzed. The zinc in the alloy was reacted with 35.123g of HC...
Questions




Mathematics, 02.12.2020 03:30

Mathematics, 02.12.2020 03:30

Chemistry, 02.12.2020 03:30

Mathematics, 02.12.2020 03:30

Social Studies, 02.12.2020 03:30

Mathematics, 02.12.2020 03:30

Mathematics, 02.12.2020 03:30

Advanced Placement (AP), 02.12.2020 03:30

Biology, 02.12.2020 03:30

Mathematics, 02.12.2020 03:30

Mathematics, 02.12.2020 03:30

Mathematics, 02.12.2020 03:30

Mathematics, 02.12.2020 03:30

Mathematics, 02.12.2020 03:30


Arts, 02.12.2020 03:30

Computers and Technology, 02.12.2020 03:30

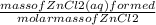
 = 0.0087 moles
= 0.0087 moles