3. Based on the following balanced equation:
2 C H20 + 13 02 8 CO2 + 10 H2O
a. How many moles...

Chemistry, 15.08.2021 01:00 lorraneb31
3. Based on the following balanced equation:
2 C H20 + 13 02 8 CO2 + 10 H2O
a. How many moles of O2 are required to react completely with 4.00 moles of C4H10?
3.00 6.00 8.00 10.00 13.00 16.00 20,00 23,00 26.00
b. How many moles of CO2 are produced after complete reaction of 4.00 moles of C H10?
3.00 6.00 8.00 10.00 13,00 16.00 20.00 23.00 26.00
c. How many moles of H2O are produced after complete reaction of 4.00 moles of C&H10?
3.00 6.00 8.00 10.00 13.00 1.6.00 20.00 23,00 26.00

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
You know the right answer?
Questions





Mathematics, 13.06.2021 01:20

Computers and Technology, 13.06.2021 01:20

History, 13.06.2021 01:20

Social Studies, 13.06.2021 01:20












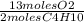 = 26 moles O2
= 26 moles O2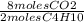 = 16 moles CO2
= 16 moles CO2 = 20 moles H20
= 20 moles H20

