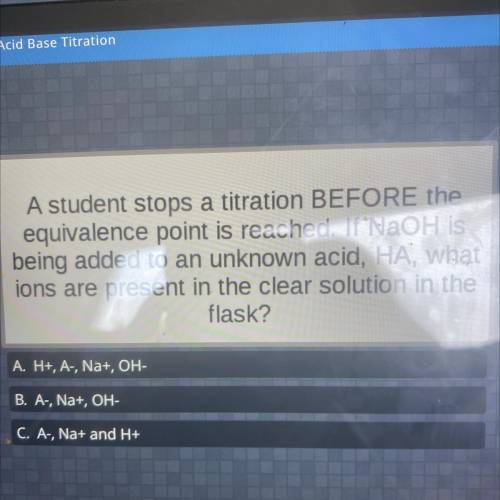
A student stops a titration BEFORE the
equivalence point is reached. If NaOH is
being added t...


Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
Questions




Mathematics, 31.07.2021 08:10



Mathematics, 31.07.2021 08:10


Mathematics, 31.07.2021 08:10

Mathematics, 31.07.2021 08:10

Engineering, 31.07.2021 08:10

Mathematics, 31.07.2021 08:10

History, 31.07.2021 08:10

Social Studies, 31.07.2021 08:10



Mathematics, 31.07.2021 08:10



Mathematics, 31.07.2021 08:10