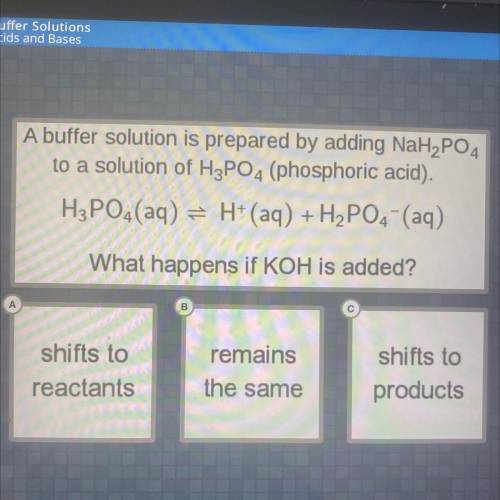
to a solution of H3PO4 (phosphoric acid).

Chemistry, 15.08.2021 03:50 lolomgwtfnvm4
A buffer solution is prepared by adding NaH2PO4
to a solution of H3PO4 (phosphoric acid).
H3PO4 (aq) = H+ (aq) + H2PO4 (aq)
What happens if KOH is added?
remains
shifts to
reactants
shifts to
products
the same

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3

Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3

Chemistry, 23.06.2019 17:00
During which of the following phases of the moon do we see the left half of the moon as lit? full moon first quarter moon gibbous moon third quarter moon any is greatly : )
Answers: 1
You know the right answer?
A buffer solution is prepared by adding NaH2PO4
to a solution of H3PO4 (phosphoric acid).
to a solution of H3PO4 (phosphoric acid).
Questions

History, 13.07.2019 00:30


Mathematics, 13.07.2019 00:30

Spanish, 13.07.2019 00:30







History, 13.07.2019 00:30


Mathematics, 13.07.2019 00:30

Mathematics, 13.07.2019 00:30



Physics, 13.07.2019 00:30

Health, 13.07.2019 00:30

Computers and Technology, 13.07.2019 00:30

Computers and Technology, 13.07.2019 00:30



