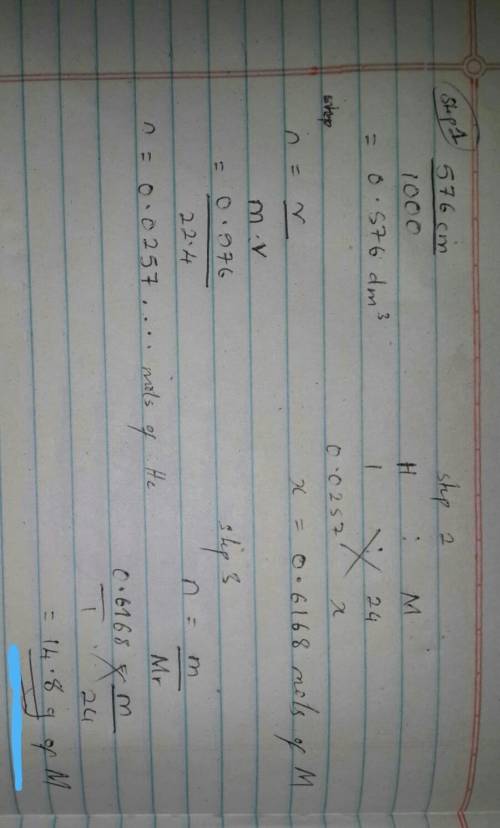Chemistry, 17.08.2021 14:00 laniflower6
An excess of a divalent metal M was dissolved in a limited volume of hydrocloric acid. If 576cm³ of hydrogen was librated at s. t.p , what was the mass of metal that produced this volume of gydrogen?( M = 24, H = 1 , molar volume of gas at s. t.p = 22.4dm³)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1


Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
An excess of a divalent metal M was dissolved in a limited volume of hydrocloric acid. If 576cm³ of...
Questions










Physics, 18.01.2021 14:00

Mathematics, 18.01.2021 14:00


Computers and Technology, 18.01.2021 14:00

Mathematics, 18.01.2021 14:00



Social Studies, 18.01.2021 14:00