
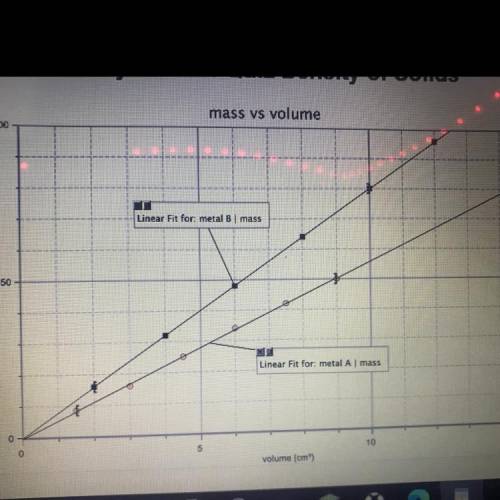
A student graphed the following data:
1. Based on this graph, how does metal B differ from metal A?
2. What is the density of metal B? Show all your work and include appropriate units.
3. What is the mass of 9.0cm^3 of metal B? Find this in two different ways.
a. Mark on the above graph how you might determine this.
b. Show how you could calculate this mathematically.
(HELP WITH THESE QUESTIONS PLEASE!!)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
In a laboratory experiment, a fermenting aqueous solution of glucose and yeast produces carbon dioxide gas and ethanol. the solution was heated by burning natural gas in a bunsen burner to distill the ethanol that formed in the flask. during the distillation, the ethanol evaporated and then condensed in the receiving flask. the flame of the burner was kept too close to the bottom of the flask and some of the glucose decomposed into a black carbon deposit on the inside of the flask. during this experiment the following changes occurred. which of these changes involved a physical change and not a chemical change? check all that apply. 1-condensation of ethanol 2-evaporation of ethanol 3- formation of carbon dioxide gas from glucose burning of natural gas 4-formation of ethanol from glucose by yeast 5-formation of a carbon deposit inside the flask
Answers: 2

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
A student graphed the following data:
1. Based on this graph, how does metal B differ from metal A...
Questions

English, 27.12.2021 23:40

SAT, 27.12.2021 23:40


Computers and Technology, 27.12.2021 23:40



Advanced Placement (AP), 27.12.2021 23:40


English, 27.12.2021 23:40


Business, 27.12.2021 23:40


Chemistry, 27.12.2021 23:40


English, 27.12.2021 23:40



SAT, 27.12.2021 23:40


SAT, 27.12.2021 23:40



