
Chemistry, 27.08.2021 08:10 ayoismeisalex
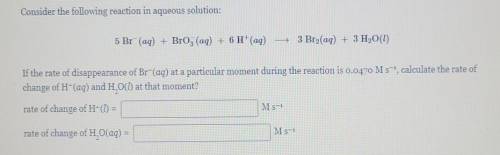
Consider the following reaction in aqueous solution: 5 Br (aq) + Brog (aq) + 6 H+(aq) - 3 Bro(aq) + 3 H2O(l) If the rate of disappearance of Br-(aq) at a particular moment during the reaction is 0.0470 M s-, calculate the rate of change of H-(aq) and H. O() at that moment? rate of change of H- = M s-1 rate of change of H. O(aq) = M s-1

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
Consider the following reaction in aqueous solution: 5 Br (aq) + Brog (aq) + 6 H+(aq) - 3 Bro(aq) +...
Questions


English, 23.07.2019 23:00




Mathematics, 23.07.2019 23:00

Biology, 23.07.2019 23:00







Social Studies, 23.07.2019 23:00



English, 23.07.2019 23:00



History, 23.07.2019 23:00



