How many significant figures are there in each of the following measured values?
a. 6.002 cm
...

Chemistry, 27.08.2021 08:50 paulethjara
How many significant figures are there in each of the following measured values?
a. 6.002 cm
b. 0.0020 m
c. 10.0500
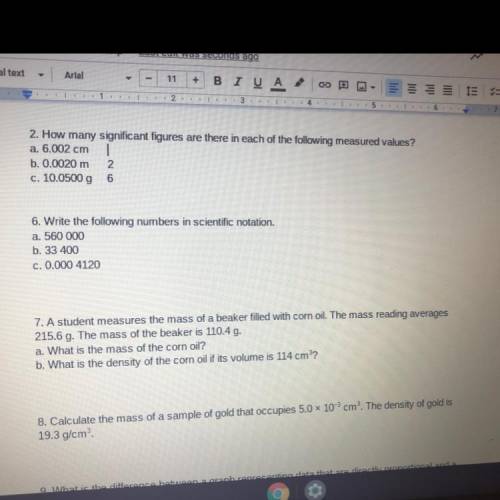

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1


Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
Questions



Mathematics, 04.12.2020 19:20


History, 04.12.2020 19:20



English, 04.12.2020 19:20



Advanced Placement (AP), 04.12.2020 19:20




Mathematics, 04.12.2020 19:20

Computers and Technology, 04.12.2020 19:20

Physics, 04.12.2020 19:20


Mathematics, 04.12.2020 19:20



