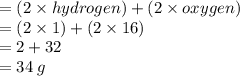Chemistry, 27.08.2021 16:20 cutegirl0987
Hich formula can be used to calculate the molar mass of hydrogen peroxide (H2O2)? (5 points)
Select one:
a. molar mass of H + molar mass of O
b.2 x molar mass of H + molar mass of O
c. molar mass of H + 2 x molar mass of O Incorrect
d.2 x molar mass of H + 2 x molar mass of O
Which of the following statements best defines the actual yield of a reaction? (5 points)
Select one:
a. The amount of product measured after a reaction
b. The ratio of measured yield over theoretical yield
c. The maximum amount of product that can be obtained Incorrect
d. The ratio of measured yield over stoichiometric yield

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:00
How are isotopes of the same chemical element alike? how are they different?
Answers: 1

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

You know the right answer?
Hich formula can be used to calculate the molar mass of hydrogen peroxide (H2O2)? (5 points)
Selec...
Questions

English, 02.07.2019 15:30

Physics, 02.07.2019 15:30

Mathematics, 02.07.2019 15:30

Social Studies, 02.07.2019 15:30



Mathematics, 02.07.2019 15:30


Health, 02.07.2019 15:30

Mathematics, 02.07.2019 15:30

English, 02.07.2019 15:30

Computers and Technology, 02.07.2019 15:30

English, 02.07.2019 15:30

Mathematics, 02.07.2019 15:30


Mathematics, 02.07.2019 15:30


Mathematics, 02.07.2019 15:30


English, 02.07.2019 15:30