
Chemistry, 30.08.2021 04:30 khikhi1705
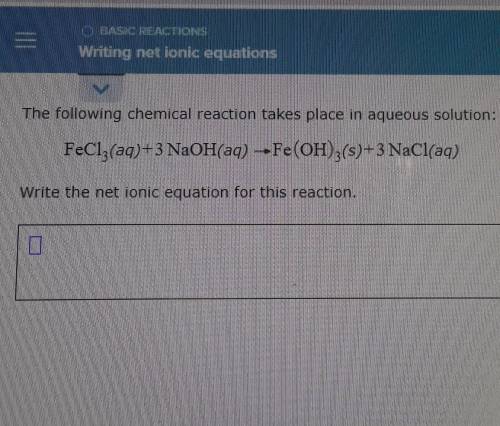
The following chemical reaction takes place in aqueous solution: FeCl3(aq)+3 NaOH(aq) --Fe(OH)3(s)+3 NaCl(aq) Write the net ionic equation for this reaction.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
You know the right answer?
The following chemical reaction takes place in aqueous solution: FeCl3(aq)+3 NaOH(aq) --Fe(OH)3(s)+3...
Questions

Mathematics, 18.06.2021 23:30





English, 18.06.2021 23:30

SAT, 18.06.2021 23:30




English, 18.06.2021 23:30

Mathematics, 18.06.2021 23:30



Spanish, 18.06.2021 23:30



Computers and Technology, 18.06.2021 23:30


English, 18.06.2021 23:30



