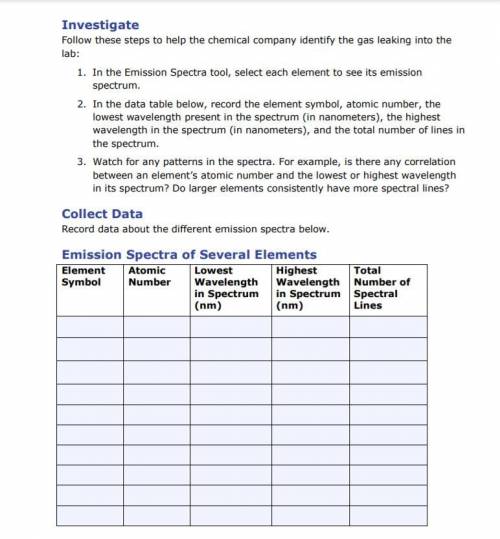
1. In the Emission Spectra tool, select each element to see its emission
spectrum.
2. In th...

1. In the Emission Spectra tool, select each element to see its emission
spectrum.
2. In the data table below, record the element symbol, atomic number, the
lowest wavelength present in the spectrum (in nanometers), the highest
wavelength in the spectrum (in nanometers), and the total number of lines in
the spectrum.
3. Watch for any patterns in the spectra. For example, is there any correlation
between an element’s atomic number and the lowest or highest wavelength
in its spectrum? Do larger elements consistently have more spectral lines?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1
You know the right answer?
Questions


Mathematics, 26.02.2021 22:40


Mathematics, 26.02.2021 22:40


English, 26.02.2021 22:40

Mathematics, 26.02.2021 22:40


Social Studies, 26.02.2021 22:40

Mathematics, 26.02.2021 22:40

Biology, 26.02.2021 22:40

Mathematics, 26.02.2021 22:40




History, 26.02.2021 22:40

Mathematics, 26.02.2021 22:40

Mathematics, 26.02.2021 22:40


Social Studies, 26.02.2021 22:40



