
Chemistry, 02.09.2021 07:00 minecraft37385
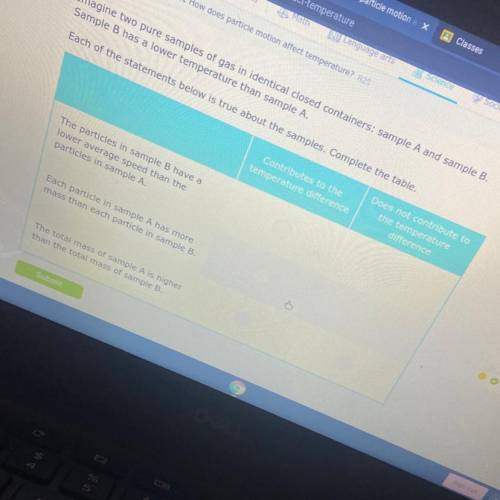
Imagine two pure samples of gas in identical closed containers: sample A and sample B.
Sample B has a lower temperature than sample A.
Each of the statements below is true about the samples. Complete the table.
Contributes to the
temperature difference
Does not contribute to
the temperature
difference
The particles in sample B have a
lower average speed than the
particles in sample A.
Each particle in sample A has more
mass than each particle in sample B.
The total mass of sample A is higher
than the total mass of sample B.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2


Chemistry, 23.06.2019 15:00
Solve this problem using the appropriate law. (remember that ) what is the pressure of 1.9 mols of nitrogen gas in a 9.45 l tank and at a temperature of 228 k?
Answers: 1
You know the right answer?
Imagine two pure samples of gas in identical closed containers: sample A and sample B.
Sample B ha...
Questions

History, 02.12.2020 03:00

English, 02.12.2020 03:00

Mathematics, 02.12.2020 03:00


Mathematics, 02.12.2020 03:00


Geography, 02.12.2020 03:00



Chemistry, 02.12.2020 03:00

Mathematics, 02.12.2020 03:00



History, 02.12.2020 03:00

Mathematics, 02.12.2020 03:00



Mathematics, 02.12.2020 03:00






