
Chemistry, 02.09.2021 17:50 brainewashed11123
It is easier to determine the electron configurations for the p-block elements in periods 1, 2, and 3 than to determine the electron configurations for the rest of the p-block elements in the periodic table because
they have a larger number of core electrons.
their electrons are assigned to s and p orbitals only.
their electrons are placed in a higher number of orbitals.
they have more valence electrons available for bonding.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 23.06.2019 00:30
When a beta particle is emitted, the mass number of the nucleus a. decreases by one b. increases by one c. remains the same d. decreases by two
Answers: 2

Chemistry, 23.06.2019 10:00
The image shows the process of which is used in nuclear power plants. photo attached
Answers: 1

Chemistry, 23.06.2019 13:30
What is one measurement needed to calculate the speed of an object?
Answers: 1
You know the right answer?
It is easier to determine the electron configurations for the p-block elements in periods 1, 2, and...
Questions




Mathematics, 26.10.2020 01:00

Chemistry, 26.10.2020 01:00




Mathematics, 26.10.2020 01:00

Mathematics, 26.10.2020 01:00



Mathematics, 26.10.2020 01:00

Social Studies, 26.10.2020 01:00


Mathematics, 26.10.2020 01:00


Mathematics, 26.10.2020 01:00


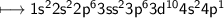
![\\ \sf\longmapsto [Ar]3d^{10}4s^24p^1](/tpl/images/2453/0445/fccbb.png)


