
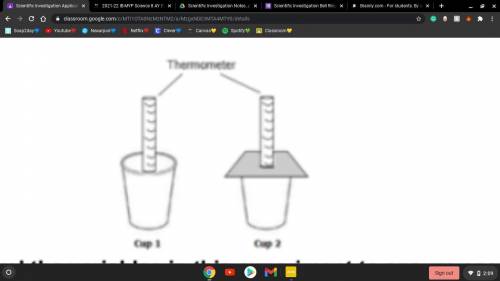
Cody wanted to know how much heat was released from a reaction of steel wool and
vinegar. He placed a thermometer inside a cup that held the steel wool and vinegar. He must decide whether to leave the cup open, as shown with Cup 1, or to cover the cup, as shown with Cup 2.
Which design would control the variables in this experiment to ensure accurate
temperature readings?
A) Cup 1. The thermometer can be read more accurately.
B) Cup 1. It will not trap the heat and alter the temperature readings.
C) Cup 2. The cover will control for any loss of heat from the top of the cup.
D) Cup 2. It will prevent fumes from escaping into the air.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

You know the right answer?
Cody wanted to know how much heat was released from a reaction of steel wool and
vinegar. He place...
Questions



English, 11.04.2021 01:00


Mathematics, 11.04.2021 01:00


Advanced Placement (AP), 11.04.2021 01:00




History, 11.04.2021 01:00


Arts, 11.04.2021 01:00


Mathematics, 11.04.2021 01:00


Arts, 11.04.2021 01:00

Mathematics, 11.04.2021 01:00

Spanish, 11.04.2021 01:00

History, 11.04.2021 01:00



