
Chemistry, 04.09.2021 16:30 mairealexander87
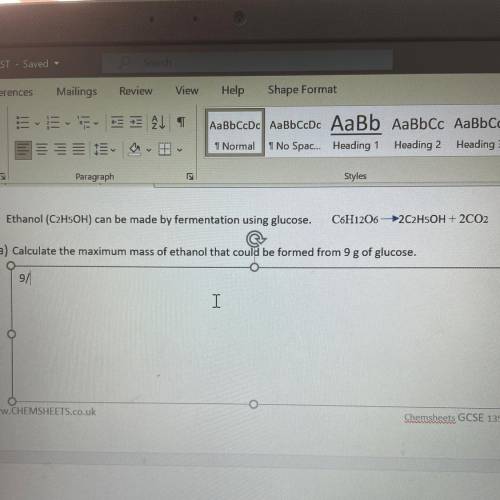
Ethanol (C2H5OH)can be made by fermentation using glucose. C6H12O6 –>2C2H5OH+2CO2 Calculate the maximum mass of ethanol that could be formed from 9g of glucose.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1


Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
Ethanol (C2H5OH)can be made by fermentation using glucose. C6H12O6 –>2C2H5OH+2CO2
Calculate the...
Questions








History, 19.11.2019 18:31




Social Studies, 19.11.2019 18:31


Chemistry, 19.11.2019 18:31

English, 19.11.2019 18:31


Advanced Placement (AP), 19.11.2019 18:31





