***ASAP*
(I know the answer just can't figure out how to put in box(s)
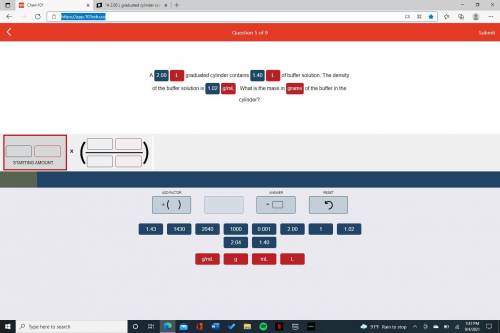
A 2.00 L graduat...

Chemistry, 05.09.2021 04:00 aliami0306oyaj0n
***ASAP*
(I know the answer just can't figure out how to put in box(s)
A 2.00 L graduated cylinder contains 1.40 L of buffer solution. The density of the buffer solution is 1.02 g/mL. What is the mass in grams of the buffer in the cylinder?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1


Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2
You know the right answer?
Questions




Mathematics, 20.04.2020 19:53

Social Studies, 20.04.2020 19:53



Biology, 20.04.2020 19:53





Social Studies, 20.04.2020 19:53

Health, 20.04.2020 19:53

Mathematics, 20.04.2020 19:53

Social Studies, 20.04.2020 19:53




Computers and Technology, 20.04.2020 19:53



