
Chemistry, 06.09.2021 05:50 destiny465
Use the References to access important values if needed for this question.
H
A-Z
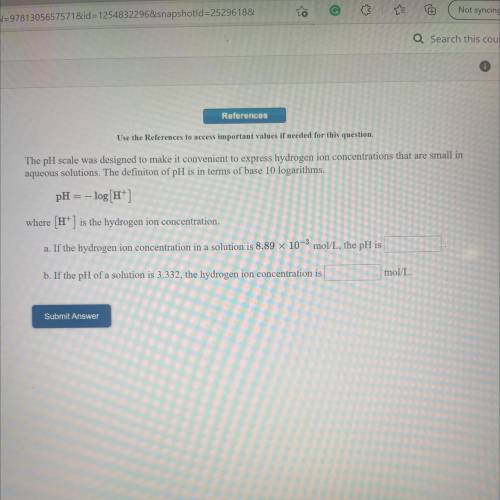
The pH scale was designed to make it convenient to express hydrogen ion concentrations that are small in
aqueous solutions. The definiton of pH is in terms of base 10 logarithms.
pH = -log[H+]
where (H+) is the hydrogen ion concentration.
a. If the hydrogen ion concentration in a solution is 8.89 x 10-3 mol/L, the pH is
b. If the pH of a solution is 3.332, the hydrogen ion concentration is
mol/L.
Submit Answer

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3
You know the right answer?
Use the References to access important values if needed for this question.
H
A-Z
The p...
A-Z
The p...
Questions


Mathematics, 25.05.2021 17:50


Mathematics, 25.05.2021 17:50

Mathematics, 25.05.2021 17:50


Spanish, 25.05.2021 17:50




History, 25.05.2021 17:50

Chemistry, 25.05.2021 17:50




World Languages, 25.05.2021 17:50


Mathematics, 25.05.2021 17:50

Mathematics, 25.05.2021 17:50

Health, 25.05.2021 17:50



