
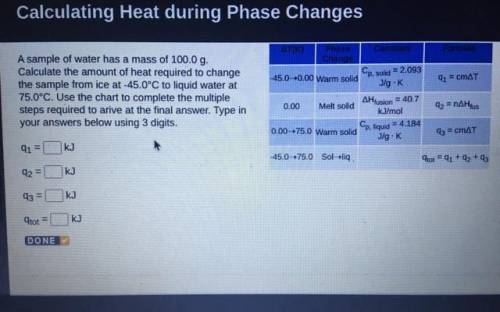
A sample of water has a mass of 100.0 g.
Calculate the amount of heat required to change
the sample from ice at -45.0°C to liquid water at
75.0°C. Use the chart to complete the multiple
steps required to arive at the final answer. Type in
your answers below using 3 digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1
You know the right answer?
A sample of water has a mass of 100.0 g.
Calculate the amount of heat required to change
the...
the...
Questions


English, 01.02.2021 23:00





Chemistry, 01.02.2021 23:00

Mathematics, 01.02.2021 23:00

Social Studies, 01.02.2021 23:00






Social Studies, 01.02.2021 23:00





Mathematics, 01.02.2021 23:00



