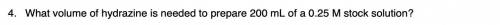Please provide a step by step solution
...

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
Questions

History, 11.12.2021 05:10


Mathematics, 11.12.2021 05:20


Mathematics, 11.12.2021 05:20


English, 11.12.2021 05:20

Mathematics, 11.12.2021 05:20

Mathematics, 11.12.2021 05:20

Spanish, 11.12.2021 05:20

Mathematics, 11.12.2021 05:20



Mathematics, 11.12.2021 05:20

Mathematics, 11.12.2021 05:20

Chemistry, 11.12.2021 05:20

Mathematics, 11.12.2021 05:20

Chemistry, 11.12.2021 05:20

Mathematics, 11.12.2021 05:20