Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
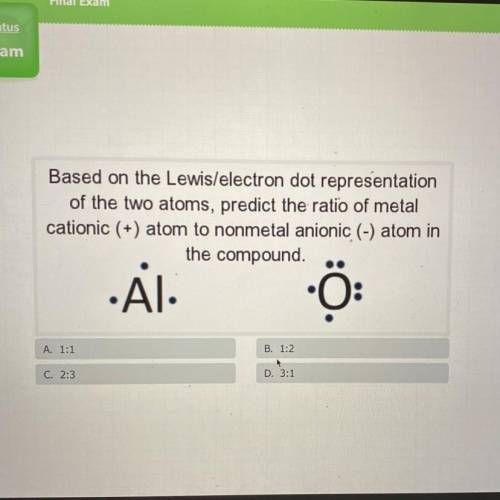
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
...
...
Questions

Mathematics, 13.10.2019 18:30


Health, 13.10.2019 18:30


Mathematics, 13.10.2019 18:30

Chemistry, 13.10.2019 18:30


Computers and Technology, 13.10.2019 18:30


Mathematics, 13.10.2019 18:30


Health, 13.10.2019 18:30



Mathematics, 13.10.2019 18:30

Biology, 13.10.2019 18:30

Business, 13.10.2019 18:30



Biology, 13.10.2019 18:30