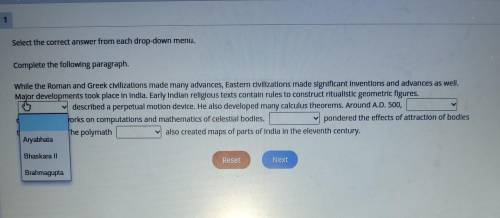
Can I please get some help on this
1st answers
A. Aryabhata
B. Bhaskara ll
C. Bra...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 23.06.2019 08:50
Reacting masses1 calcium carbonate breaks down on heating to produce calcium oxide and carbondioxide gas.caco3 + cao + co2a student heats 15 g of calcium carbonate strongly in a crucible.relative atomic masses (a): ca = 40, c = 12, o = 16.calculate the mass of calcium oxide produced by this reaction.(5 marks)
Answers: 3
You know the right answer?
Questions

Mathematics, 27.08.2020 23:01



History, 27.08.2020 23:01


Arts, 27.08.2020 23:01


Arts, 27.08.2020 23:01


English, 27.08.2020 23:01








Mathematics, 27.08.2020 23:01


Mathematics, 27.08.2020 23:01