
Chemistry, 12.09.2021 19:20 gracebrownnn
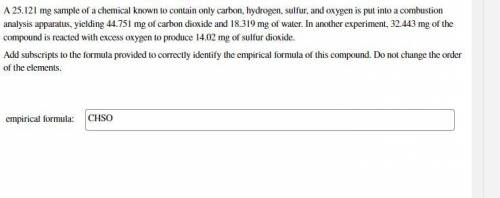
25.121 mg sample of a chemical known to contain only carbon, hydrogen, sulfur, and oxygen is put into a combustion analysis apparatus, yielding 44.751 mg of carbon dioxide and 18.319 mg of water. In another experiment, 32.443 mg of the compound is reacted with excess oxygen to produce 14.02 mg of sulfur dioxide.
Add subscripts to the formula provided to correctly identify the empirical formula of this compound. Do not change the order of the elements.
empirical formula:
CHSO

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 06:10
How can liquids be seperated by density a the liquids are absorbed onto a paper b the liquids are turned into seperate vapors c the liquids are collected as they evaporate d the liquids are allowed to seperate into layers
Answers: 1

Chemistry, 23.06.2019 12:30
What are some examples of anthropogenic atmospheric particulates?
Answers: 1

Chemistry, 23.06.2019 14:00
What is the final volume in milliliters when 0.641 l of a 34.0 % (m/v) solution is diluted to 23.5 % (m/v)?
Answers: 1

You know the right answer?
25.121 mg sample of a chemical known to contain only carbon, hydrogen, sulfur, and oxygen is put int...
Questions

English, 26.01.2021 01:00

English, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00

English, 26.01.2021 01:00


Chemistry, 26.01.2021 01:00


Mathematics, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00







Mathematics, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00





