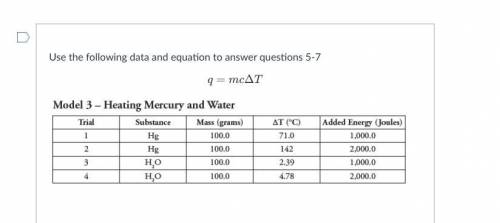
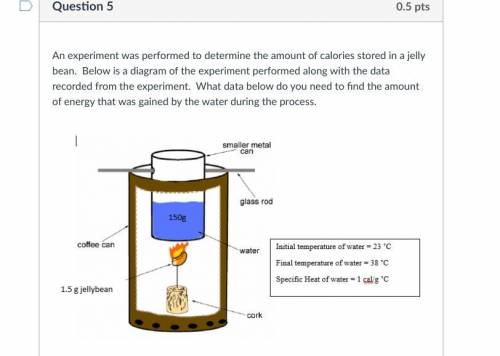
5.
A. mass of water and mass of jelly bean
B. mass of water, mass of jelly bean and ch...

Chemistry, 15.09.2021 22:50 needhelp243435
5.
A. mass of water and mass of jelly bean
B. mass of water, mass of jelly bean and change in temperature of water
C. mass of jelly bean, specific heat of water and change in temperature of water
D. mass of water, specific heat of water, and change in temperature of water
6. You can make ice cream by dissolving a chemical in ice water. By adding the chemical the temperature of water decreases causing the milk and sugar to freeze rapidly. You are tasked with designing a procedure to determine what chemical will freeze the ice cream the fastest. What piece of evidence would be best to help you select your chemical
A. The sample with the lowest initial temperature would work best
B. The sample with the highest mass will work the best
C. The sample with the smallest mass will work best
D. The sample with the most negative temperature change will work best
7. When conducting the experiment described above, What container would be the best place to mix your chemical with water? What explanation best fits?
A. Beaker, because it allows the most energy to be lost or gained by the environment
B. Styrofoam cup because it has a small mass and won't affect the mass of the water
C. Styrofoam cup because it prevents energy from being lost or gained to/from the surroundings
D. beaker because it has volume marks and will help you determine the volume of the solution

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3


Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
Questions


Mathematics, 16.04.2021 19:10










Mathematics, 16.04.2021 19:10



Mathematics, 16.04.2021 19:10


History, 16.04.2021 19:10

Chemistry, 16.04.2021 19:10


History, 16.04.2021 19:10



