Pls help I'm marking brainliest for best answer Laundry Day
Scientific Background
A chemistr...

Chemistry, 18.09.2021 23:50 tannerlynn4320
Pls help I'm marking brainliest for best answer Laundry Day
Scientific Background
A chemistry student uses powdered laundry detergent, sodium carbonate (Na2CO3), when washing clothes. She accidentally poured epsom salt, magnesium sulfate (MgSO4), in the washing machine along with the laundry detergent. When both substances dissolved in the water, the student made observations.
Develop an argument explaining if the student observed a chemical reaction.
In your essay (3 paragraphs: one for each bullet) be sure to complete the following tasks:
- State whether or not a chemical reaction occurred and justify your claim using the student’s observations.
- Identify the reactants and products and explain what the (aq) and (s) represent in the equation.
- Determine the type of reaction that occurred in the washing machine and explain.
(Documents A through D are related to this Writing Task.)
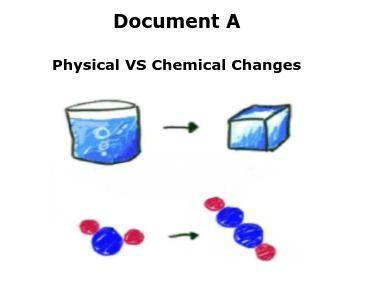
Document A
Physical VS Chemical Changes
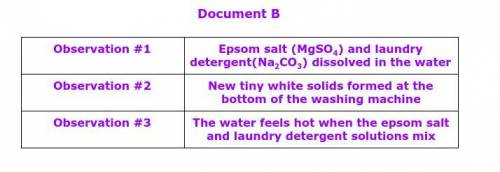
Document B
Observation #1
Epsom salt (MgSO4) and laundry detergent(Na2CO3) dissolved in the water
Observation #2
New tiny white solids formed at the bottom of the washing machine
Observation #3
The water feels hot when the epsom salt and laundry detergent solutions mix
Document C
MgSO4 (aq) + Na2CO3 (aq) → MgCO3 (s) + Na2SO4 (aq)
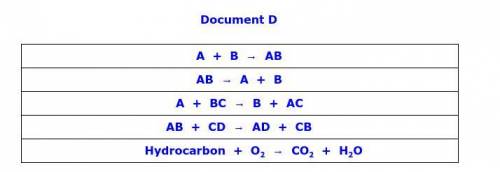
Document D
A + B → AB
AB → A + B
A + BC → B + AC
AB + CD → AD + CB
Hydrocarbon + O2 → CO2 + H2O


Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1
You know the right answer?
Questions

English, 25.07.2019 17:00

Mathematics, 25.07.2019 17:00

History, 25.07.2019 17:00



Mathematics, 25.07.2019 17:00



Mathematics, 25.07.2019 17:00

Health, 25.07.2019 17:00


Mathematics, 25.07.2019 17:00


Physics, 25.07.2019 17:00

Mathematics, 25.07.2019 17:00

Mathematics, 25.07.2019 17:00

Mathematics, 25.07.2019 17:00

Physics, 25.07.2019 17:00




