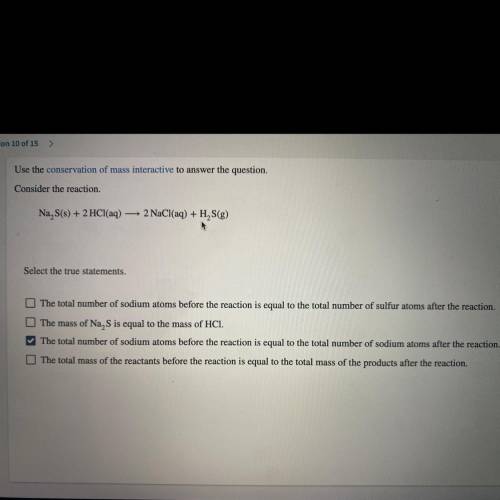
Use the conservation of mass interactive to answer the question.
Consider the reaction.
Na S...

Chemistry, 19.09.2021 06:20 Emptypockets451
Use the conservation of mass interactive to answer the question.
Consider the reaction.
Na S(s) + 2 HCl(aq) — 2 NaCl(aq) + H2S(g)
*Select the true statements.*
A. The total number of sodium atoms before the reaction is equal to the total number of sulfur atoms after the reaction.
B. The mass of Na, S is equal to the mass of HCI.
C. The total number of sodium atoms before the reaction is equal to the
total number of sodium atoms after the reaction.
D. The total mass of the reactants before the va reaction is equal to the total mass of the products after the reaction.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
Questions



Biology, 10.10.2019 16:40

Mathematics, 10.10.2019 16:40


Mathematics, 10.10.2019 16:40

Social Studies, 10.10.2019 16:40



Mathematics, 10.10.2019 16:40

Mathematics, 10.10.2019 16:40

Mathematics, 10.10.2019 16:40

History, 10.10.2019 16:40

Social Studies, 10.10.2019 16:40

Mathematics, 10.10.2019 16:40


Mathematics, 10.10.2019 16:40

Chemistry, 10.10.2019 16:40

Mathematics, 10.10.2019 16:40



