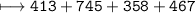Use the reaction and bond information to answer the question.
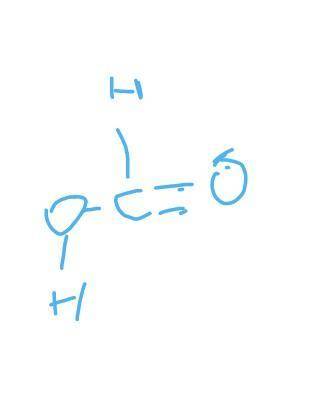
H2 + CO2 → CH2O2
Reactan...

Chemistry, 19.09.2021 14:00 CuteDoggo1828
Use the reaction and bond information to answer the question.
H2 + CO2 → CH2O2
Reactant bond energies: H–H is 432 kJ/mol, C=O is 799 kJ/mol
Product bond energies:
C–H is 413 kJ/mol, C=O is 745 kJ/mol
C–O is 358 kJ/mol, O–H is 467 kJ/mol
How much energy will be given off by the products?
1,570 kJ
1,983 kJ
1,238 kJ
1,516 kJ

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2


Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3
You know the right answer?
Questions


English, 28.06.2019 16:30

Geography, 28.06.2019 16:30


Health, 28.06.2019 16:30

Mathematics, 28.06.2019 16:30


Biology, 28.06.2019 16:30

Mathematics, 28.06.2019 16:30


Advanced Placement (AP), 28.06.2019 16:30



Mathematics, 28.06.2019 16:30


Health, 28.06.2019 16:30



History, 28.06.2019 16:30