
Chemistry, 22.09.2021 02:30 gwendallinesikes
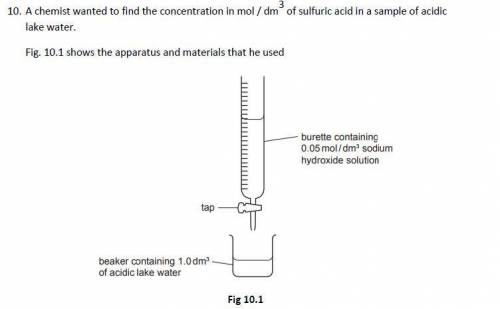
The chemist slowly added 0.05 mol / dm3 sodium hydroxide solution to 1.0 dm3 of acidic lake water contained in a beaker until the acid had just been neutralised
The chemist found that it required 12.5 cm3 of 0.05 mol / dm3 sodium hydroxide solution to neutralise the acid
a. State the number of moles of sodium hydroxide which are dissolved in 1.0 dm3 of the sodium hydroxide solution
b. Calculate the number of moles of sodium hydroxide which are dissolved in 12.5 cm3 of the sodium hydroxide solution. Show your workings.
Show your working.
c. The balanced equation for the neutralisation reaction is
2NaOH + H2SO4 → Na2SO4 + 2H2O
Calculate the number of moles of sulfuric acid which were contained in 1.0 dm3 of acidic lake water.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
The chemist slowly added 0.05 mol / dm3 sodium hydroxide solution to 1.0 dm3 of acidic lake water co...
Questions

Social Studies, 05.11.2019 00:31

History, 05.11.2019 00:31







English, 05.11.2019 00:31

History, 05.11.2019 00:31





History, 05.11.2019 00:31

Biology, 05.11.2019 00:31




History, 05.11.2019 00:31



