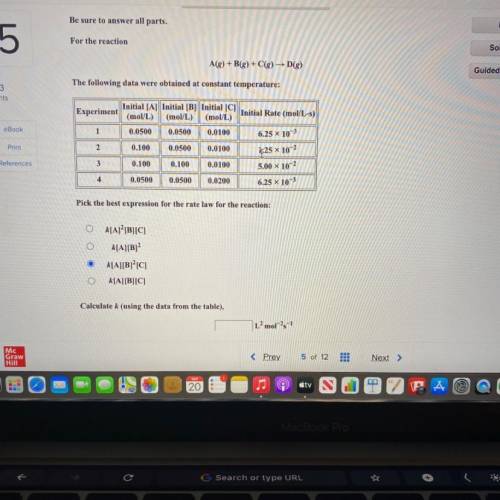
Be sure to answer all parts.
For the reaction
A(g) +B(g) + C(g) D()
The following data...

Be sure to answer all parts.
For the reaction
A(g) +B(g) + C(g) D()
The following data were obtained at constant temperature:
Initial [A] Initial [B] Initial [C]
Experiment
Initial Rate (mol/L-s)
(mol/L) (mol/L) (mol/L)
1 0.0500 0.0500 0.0100
6.25 x 103
2 0.100 0.0500 0.0100
3 0.100 0.100 0.0100 5.00 x 10-2
4 0.0500 0.0500 0.0200
6.25 x 10-3
1,25 x 10-2
Pick the best expression for the rate law for the reaction:
k[A] [B][C]
k[A][B]
k[A][B]°C]
k[A][B][C]
Calculate k (using the data from the table),
L’mol-1

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2
You know the right answer?
Questions

Biology, 22.11.2020 14:00


Mathematics, 22.11.2020 14:00




Mathematics, 22.11.2020 14:00

History, 22.11.2020 14:00


Chemistry, 22.11.2020 14:00


Advanced Placement (AP), 22.11.2020 14:00

Computers and Technology, 22.11.2020 14:00



SAT, 22.11.2020 14:00

Business, 22.11.2020 14:00


Mathematics, 22.11.2020 14:00




