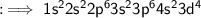The electron configuration of chromium is written as: 152 252 2p 3s 3p6 451 3d5
Is this the expected configuration? Select the correct answer and reason from the options below.
This is the expected configuration.
Both 452 3d4 and 451 3d can
represent the configuration of the
outer orbitals.
This is the expected configuration.
The expected configuration for the
outer orbitals is: 451 3d5
This is not the expected configuration.
O The expected configuration for the
outer orbitals is: 452 4p4
This is not the expected configuration.
O The expected configuration for the
outer orbitals is: 452 3d4

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

You know the right answer?
The electron configuration of chromium is written as: 152 252 2p 3s 3p6 451 3d5
Is this the expect...
Questions


Mathematics, 03.03.2021 22:20

Biology, 03.03.2021 22:20


History, 03.03.2021 22:20





Mathematics, 03.03.2021 22:20

Mathematics, 03.03.2021 22:20


Mathematics, 03.03.2021 22:20

Mathematics, 03.03.2021 22:20

Mathematics, 03.03.2021 22:20


Mathematics, 03.03.2021 22:20


Geography, 03.03.2021 22:20