CH3CO2H is a base, and H3O+ is its conjugate acid.

Chemistry, 25.09.2021 17:10 22savage2017
In the buffer solution
A)
CH3CO2H is a base, and H3O+ is its conjugate acid.
B)
H3O+ is an acid, and CH3CO2 – is its conjugate base.
C)
H3O+ is an acid, and CH3CO2H is its conjugate base.
D)
CH3CO2H is an acid, and CH3CO2 – is its conjugate base.
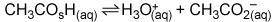

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1
You know the right answer?
In the buffer solution
A)
CH3CO2H is a base, and H3O+ is its conjugate acid.
CH3CO2H is a base, and H3O+ is its conjugate acid.
Questions



English, 12.02.2021 06:10

Mathematics, 12.02.2021 06:10

English, 12.02.2021 06:10

Mathematics, 12.02.2021 06:10


Mathematics, 12.02.2021 06:10

Mathematics, 12.02.2021 06:10

English, 12.02.2021 06:10

Health, 12.02.2021 06:10


Law, 12.02.2021 06:10



English, 12.02.2021 06:10


Mathematics, 12.02.2021 06:10

English, 12.02.2021 06:10



