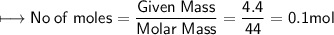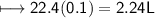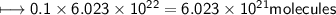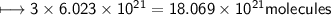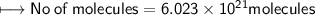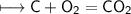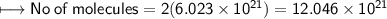Chemistry, 26.09.2021 05:00 nidiavega2009
A closed cylinder is filled with CO₂ gas. The mass of CO₂ in cylinder is 4.4 g. Now express this amount of Carbon dioxide in following terms:
a) no. of moles of CO₂ molecules
b) Volume at NTP
c) No. of 'gram molecule
d) No. of CO₂ molecules
e) No. of carbon atoms
f) No. of mole of Oxygen atoms
g) No. of molecules of oxygen

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
A closed cylinder is filled with CO₂ gas. The mass of CO₂ in cylinder is 4.4 g. Now express this amo...
Questions

History, 06.10.2019 02:30

Physics, 06.10.2019 02:30



Mathematics, 06.10.2019 02:30

History, 06.10.2019 02:30

Business, 06.10.2019 02:30

Mathematics, 06.10.2019 02:30

History, 06.10.2019 02:30

Social Studies, 06.10.2019 02:30

History, 06.10.2019 02:30

Mathematics, 06.10.2019 02:30

Mathematics, 06.10.2019 02:30


Chemistry, 06.10.2019 02:30



English, 06.10.2019 02:30


Biology, 06.10.2019 02:30