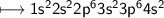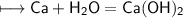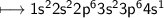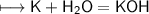Chemistry, 26.09.2021 22:10 Nickanderson21
Calcium is an element in group II of the periodic table and sits on the same period as potassium.
How would you expect the reactivity of calcium with water to compare with the reactivity of group I element with water?
Help 15 points❤️❤️

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2
You know the right answer?
Calcium is an element in group II of the periodic table and sits on the same period as potassium....
Questions











English, 08.10.2019 18:10

English, 08.10.2019 18:10

Mathematics, 08.10.2019 18:10






Mathematics, 08.10.2019 18:10

Health, 08.10.2019 18:10