For the reaction A(g)
2B(g), what is
the equilibrium concentration of B if the
initial...

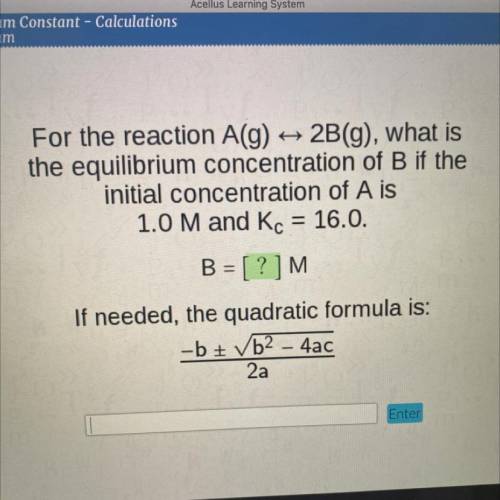
For the reaction A(g)
2B(g), what is
the equilibrium concentration of B if the
initial concentration of A is
1.0 M and Kc = 16.0.
B = [?]M
If needed, the quadratic formula is:
-b + b2 - 4ac
2a
PLEASE HELP IM SO CONFUSED AND NO LESSON VIDEOS ARE HELPFULL
ILL RATE YOU BRAINLIEST

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1

Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2
You know the right answer?
Questions





Physics, 23.03.2021 17:40


English, 23.03.2021 17:40

Mathematics, 23.03.2021 17:40


Mathematics, 23.03.2021 17:40









Mathematics, 23.03.2021 17:40

Computers and Technology, 23.03.2021 17:40



